Diagnosis within rheumatology
The diagnosis of axial SpA is complex, whereby individual symptoms or tests in isolation are insufficient to either diagnose or rule out axial SpA; rather a combination of axial SpA symptoms, physical examination, appropriate diagnostic tests and imaging should lead to diagnosis.
Explore key publications
Diagnosing axial SpA in rheumatology
Dr Antoni Chan is a Consultant Rheumatologist and Associate Medical Director at the Royal Berkshire NHS Foundation Trust.
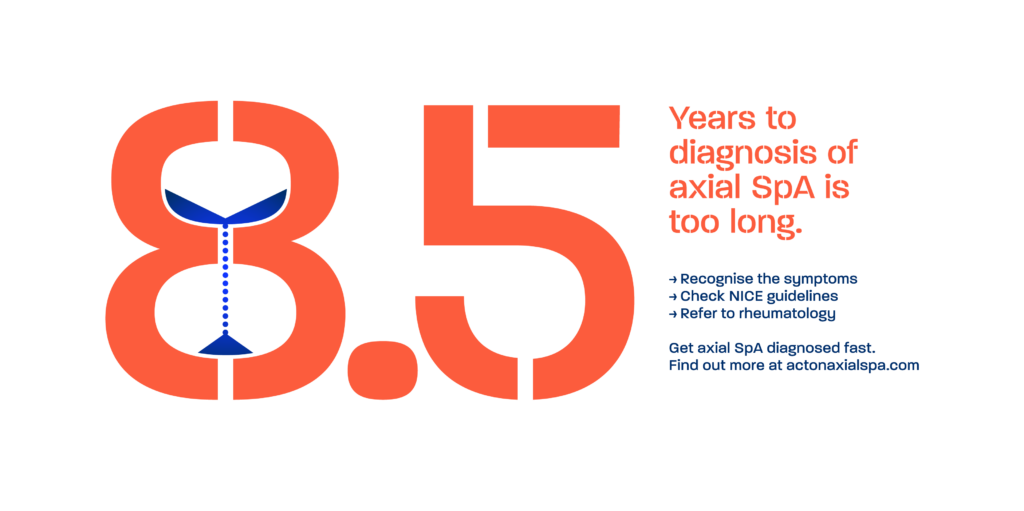
In the UK, it currently takes an average of 8.5 years for a patient to be diagnosed with axial spondyloarthritis (axial SpA). With the Gold Standard time to diagnosis programme, our goal with NASS is to reduce this to just one year. In order to do this, it is critical for us to detect symptoms of axial SpA much earlier on in the condition. In rheumatology, we must be confident in how to further investigate and interpret these symptoms in order to consider a potential diagnosis.
“The main principles of the diagnostic approach can be clearly defined: consider the pre-test probability of the disease, evaluate positive and negative results of the diagnostic test, exclude other entities, and estimate the probability of the disease at the end.”*
*Denis Poddubnyy, Classification vs diagnostic criteria: the challenge of diagnosing axial spondyloarthritis, Rheumatology, Volume 59, Issue Supplement_4, October 2020, Pages iv6–iv17, https://doi.org/10.1093/rheumatology/keaa250
Patient history - clinical features of axial SpA
The prevalence of axial SpA is 1 in 200 in the adult population. In patients attending clinic with chronic back pain, 1 in 20 of these patients will likely have axial SpA. Often, it can be mixed up with mechanical back pain. However, we must be careful to take a history to understand any prior features of inflammatory back pain. For patients with inflammatory back pain, the likelihood of axial SpA raises to ~30%.
In the presence of inflammatory back pain, we should therefore also be looking out for any other clinical features of axial SpA. Such as inflammatory bowel disease, uveitis, enthesitis and psoriasis. When these clinical features are present, especially with a family history of SpA, then we should investigate this further. The NICE Guidance NG65 provides useful referral criteria for making a diagnosis of axial spondyloarthritis (Spondyloarthritis: diagnosis and management: summary of NICE guidance | The BMJ).
Tests and investigations – although negative results do not rule out a diagnosis
Tests that can be useful are HLA-B27 and inflammatory markers such as CRP, in addition to X-ray of the sacroiliac joints. However, the HLA-B27 result may be negative, and CRP normal. The X-ray may also be normal in the early stages of disease. These negative results do not rule out a diagnosis of axial SpA. It is also important to note that axial SpA has an equal prevalence in males and females. It is no longer thought of as a male only, or a male predominant disease.
MRI and close collaboration with radiologists
If following negative X-ray or blood tests clinical suspicion remains high for axial SpA based on the physical examination and presence of other axial SpA features, an MRI scan of the whole spine and sacroiliac joints is recommended to detect any early inflammatory changes. Here, it is critical to collaborate closely with musculoskeletal radiologists, to facilitate correct interpretation and earlier identification. Please refer to the consensus BRITSpA papers on imaging in axial SpA for more information.
Making a diagnosis
Axial SpA remains a clinical diagnosis – in the presence of supporting investigations and clinical features, and absence of a more likely differential diagnosis, one can make a diagnosis of axial SpA. The diagnostic process can be thought of as a “diagnostic scale”*, weighing positive test results and present clinical features against negative test results and the presence of an alternative explanation for the symptoms. Even when all tests have been conducted, a diagnosis of axial SpA may remain uncertain. However, we know that these conditions change and evolve over time. If a diagnosis of axial SpA cannot be confirmed but clinical suspicion remains high, consider a follow‑up MRI to monitor for any potential future inflammatory changes.
It is also important to note that classification criteria such as the Assessment of Spondyloarthritis International Society (ASAS) criteria exist for axial SpA. However, classification criteria should not be applied for diagnosis. They should only be applied to identify a homogenous population of patients, for example during clinical trials, when it is useful to standardize the comparison between patients who already have an established diagnosis. See the recent publication by Denis Poddubnyy* which discusses the distinction between the clinical diagnostic approach versus the use of classification criteria to define a homogenous population for clinical trials.
Collaboration
In order to achieve the Gold Standard of reducing delays to diagnosis to 1 year, there is an important and urgent need for rheumatologists to collaborate with other specialties such as dermatology, gastroenterology, ophthalmology and radiology. This collaboration should also include GPs, primary care, physiotherapists and first contact practitioners. We need to change the narrative and move to the new standard of diagnosis of axial SpA within a year of symptom onset.

ACT ON AXIAL SpA:
A Gold Standard time to diagnosis
The current time to diagnosis of axial SpA in the UK averages approximately 8.5 years from symptom onset. This delay is unacceptable and has serious consequences for the patient. Our act on axial SpA campaign sets out a roadmap for reducing the time from symptom onset to diagnosis to just one year.
